
What was it about the early earth that first breathed life into lifeless matter? Are we unique, exceedingly rare, or was our planet but one in a million hatcheries scattered across the universe? These are questions to ponder, simmer and sin over. These are questions to discuss with good friends over good wine over good tasty dead life-forms in a good restaurant. These are questions to which we, for the first time in our history, now have sensible answers to. No longer is it deemed necessary, or even respectable, to invoke fantastical gods, talismans, shamens, and fairy tales for answers. We have a wonderful tool at our disposal - a tool the ancients lacked, a tool which today is taught in all good schools throughout the world. A tool called chemistry.

The Origin of Life, as we shall see is a chemical problem. True, classroom chemistry, as taught today by boring dusty old teachers in dilapidated damp-cold laboratories, can extinguish (like blowing out a candle) the curiosity of many a bright and promising student. Students are told to memorise staid formulas, molarities and learn the Periodic Table - by rote. Hardly, an exciting endeavour to entice the curious amongst us into a world of science. But there are a few dedicated souls amongst us who do devote their lives to science, who look beyond the formulas and staid equations. Who breathe new life into the equations. Don't worry, I won't be throwing equations at you, but I will expect you to understand and appreciate the one's I do fling in your general direction.
But first before we begin, a little warning. We can never know for sure how life really started on earth. Even if we succeed in producing bacteria that crawl out of a test tube from a swirling soup of chemicals, we will never know if that is how life actually started on our planet. All we can say is that such things are possible. The quest for the Origin of Life is not about answers to what happened at a particular moment in the year 3,851 million BC, but it is a quest for the general rules that must govern the emergence of any life, anywhere in the universe, and especially on our planet, the only example we know of where life exists. I believe the rules that apply to our planet also apply to life elsewhere - wherever it may be found in the universe. The story we’ll trace is almost certainly not correct in every detail, but it is broadly accurate, and as we’ll see, perhaps almost inevitable once certain conditions are met. It is a story of chemistry and the ancient past. It is a story of many teenage years spent in angst mulling over lifes mysteries. It is a story of Life, the Universe and Everything. It is also very exciting. I'm excited. So let's begin!
There is a fundamental rule that governs all life on earth. All living things need to generate energy in order to build bodies and survive. Without energy life reverts back to the state of lifeless inanimate matter from whence it came. Most creatures on earth are consumers and get their energy from other living things - by eating them. Every time you eat a chicken drumstick a la KFC style, you are absorbing the energy stored by the chicken in its flesh. Where did the chicken gets its energy from? From seeds. It spent hours and hours converting seeds into chicken flesh. A chicken is a fascinating device. Through its mouth enter the seeds, and down its oesophagus they go into its tummy. Eventually via a complex cogwheel of reactions called 'respiration' these seeds are converted into tasty yummy chicken flesh. The chicken converts the seeds into feathers too, and muscles, and guts, and feet, and a brain and nervous system (albeit a very small one). And the seeds? Where did they get their energy from? Or to pose the question in another way: where does the energy trapped inside all living things ultimately come from? Answer: the sun. It is the green plants and other so called 'producers' that harvest the energy in the sun to build organic carbon molecules with which to build bodies. The equation is that of photosynthesis:
6 CO2 + 6 H2O 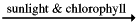 C6H12O6+ 6 O2
C6H12O6+ 6 O2
carbon dioxide + water ------ (energy from the sun absorbed by leaves) = glucose + oxygen
Let's stop and think what is happening here. Plants are effectively stripping away the hydrogen atoms in water, and then recombining them with the carbon and oxygen atoms in carbon dioxide to build glucose. From glucose they then make bodies and power their myriad functions (this is a little simplistic but it is essentially what is happening). The energy to power the combination of hydrogen with carbon dioxide comes from sunlight. The sunlight is trapped by the leaves by the green pigment chlorophyll.
The first life on earth had to be a producer. It couldn't have been a consumer; i.e. it couldn't have fed on other life, because there was no other life to feed on. At first the only producers that scientists knew of were green plants and photosynthetic bacteria. But this painted a paradox. You see the problem is that photosynthesis is a wonderful process. In fact it is too wonderful! And it is wonderful precisely because it is very efficient and very complicated (as any 1st year biochemistry student will attest to) - and therein lies the problem. It requires organelles (tiny spherical green bodies) inside cells called chloroplasts with an origami network of twisted internal membranes. It is inside chloroplasts that the reactions of photosynthesis take place and it is is an extremely complex biochemical process. It involves a chain of protein molecules embedded in the membranes. It involves a vast array of protein catalysts called enzymes to help and control these reactions. It requires a green pigment called chlorophyll to trap the light, and it requires molecules to shuttle electrons along the complexes at break neck speed. In short photosynthesis as we know it is far far too complicated to have arisen in the first life. It is way too complicated. See image below:

(Phosynthesis is far too complicated to have arisen in the first life forms)
The first life that arose, therefore had to be, by definition, very simple. It would have had to generate energy from a very simple process not involving enzymes and proteins and membranes. In short it couldn't have photosynthesised. So how did it generate it's energy? And more importantly: what did it look like? The answer as you'll see is a strange and wonderful one. In fact, the first life probably did not look like life as we understand it. It looked almost alien. To find it we must travel deep down into the ocean depths...far down, on the sea bed where strange things lurk...
It was in the early 1970s when the first pieces of the jigsaw puzzle were found. We've been putting them together ever since. The first clues to an answer to the origin of life came in the early 1970s, when rising plumes of hot water were noticed along the Galapagos Rift, not far from the Galapagos Islands . Little happened for a few years and then in 1977 a submarine descended to the rift seeking the source of the hot plume of water. What it found were hydrothermal vents; huge 80 metre tall chimneys at the bottom of the ocean venting black smoke and hot water into the cold salty sea water. What surprised everyone even more was the existence of giant tube worms, some of them eight feet long, mixed with clams and mussels, all living off the heat and minerals from the deep dark undersea vents many miles below the waters surface. The sheer abundance of life down below was astonishing; a veritable cornucopia of life approaching the exuberance of rainforest's and coral reefs; and all powered by the energy from the exhalations of underwater vents rather then sunlight. You see sunlight cannot penetrate these murky depths. These vents soon acquired the name ‘black smokers’ and since then over 200 ‘fields’ of black smokers; tottering as high as skyscrapers, have been found over the earths ridges, in the Atlantic, Pacific and Indian Oceans. But this is not smoke as we know it. The black stuff consists of broiling metal sulphides, hydrogen gas, ammonia, all seeping up from within the earth’s magma, reaching temperatures of 400 degrees centigrade in the crushing pressures of the ocean. The chimneys themselves are composed of sulphurous minerals, like iron pyrites, which settle out from the black smoke and amass as thick deposits over wide areas.
('black smokers' - deep sea hydrothermal vents - did life begin here?)
This bizarre and alien world seems like a vision of Hell itself and comes replete with hell fire, brimstone and the foul reek of hydrogen sulphide gas. And what lives in these conditions? Life. Large 8 foot worms with no mouths and no anuses, and eyeless shrimps swarming in countless multitudes on the ledges and cliffs below the smoking chimneys. Life doesn’t just scrimp by and earn a living here; it positively thrives in these infernal conditions. It needs the hydrogen gas, hydrogen sulphide, and ammonia to survive! On the surface of our planet this stuff would be poisonous. But not here. How can life survive down here? What is going on?
